 2020.03.05
2020.03.05Summary:The MES of the ingredient system is based on the pharmaceutical industry's work order management, process route management, material management, production process management and data collection functions for ingredients. It is stored in the database through coding and linked to information such as batch numbers and electronic supervision codes. , allowing users to trace products based on product coding information at any time.
In terms of work order management. MES automatic feeding system is required to support the manual creation of work orders and batch numbers according to the product name and production, and at the same time can accept from the factory upper ERP weighing and dosage system issued with the product name, production and batch number and other information of the work order.
In terms of process route management, MES weighing and feeding system needs to designate different process routes according to different products and batch information, and at the same time, it supports the optimization of the existing process routes on the basis of the new version of GMP and the existing production situation of the enterprise.
In terms of material management. The new GMP requires enterprises to establish a standardized material management system, so that the flow of materials is clear, controllable and traceable. MES batching machine needs to control the material input and output by coding the material, the code will be locked into the production process, thus preventing the material from mixing the batch and the material information through the coding of the way to the database, and the batch number, the electronic regulatory code and other information linked to facilitate the user at any time according to the product code, so that the user can be used in the process. The information of materials is stored in the database through coding, which is linked with batch number, electronic supervision code and other information, so that it is easy for the users to trace the products at any time according to the coded information.
In the production process control management, MES feeding station requires practical prompting staff to the current need for operation, restriction of operating privileges, control of key data in the production process, control of key operations in the production process, so as to enhance the qualification rate of the enterprise's products, so that the standardization of the enterprise's product data, the standardization of the production process.
In the field of data acquisition, MES automatic feeder should be able to collect the data of the current operation of the field dosing system through the data acquisition system, to avoid the need for staff to fill in the records manually to confirm the data value and fill in the value of the accuracy and other issues, to avoid the occurrence of errors to the maximum extent possible.
The specific program implementation of the dosage system includes the following six parts:
Basic data management and production modeling. PharmaSuite's Primary Data Management (PMC) module was utilized to provide the MES dosage system with the ability to maintain and manage primary data.
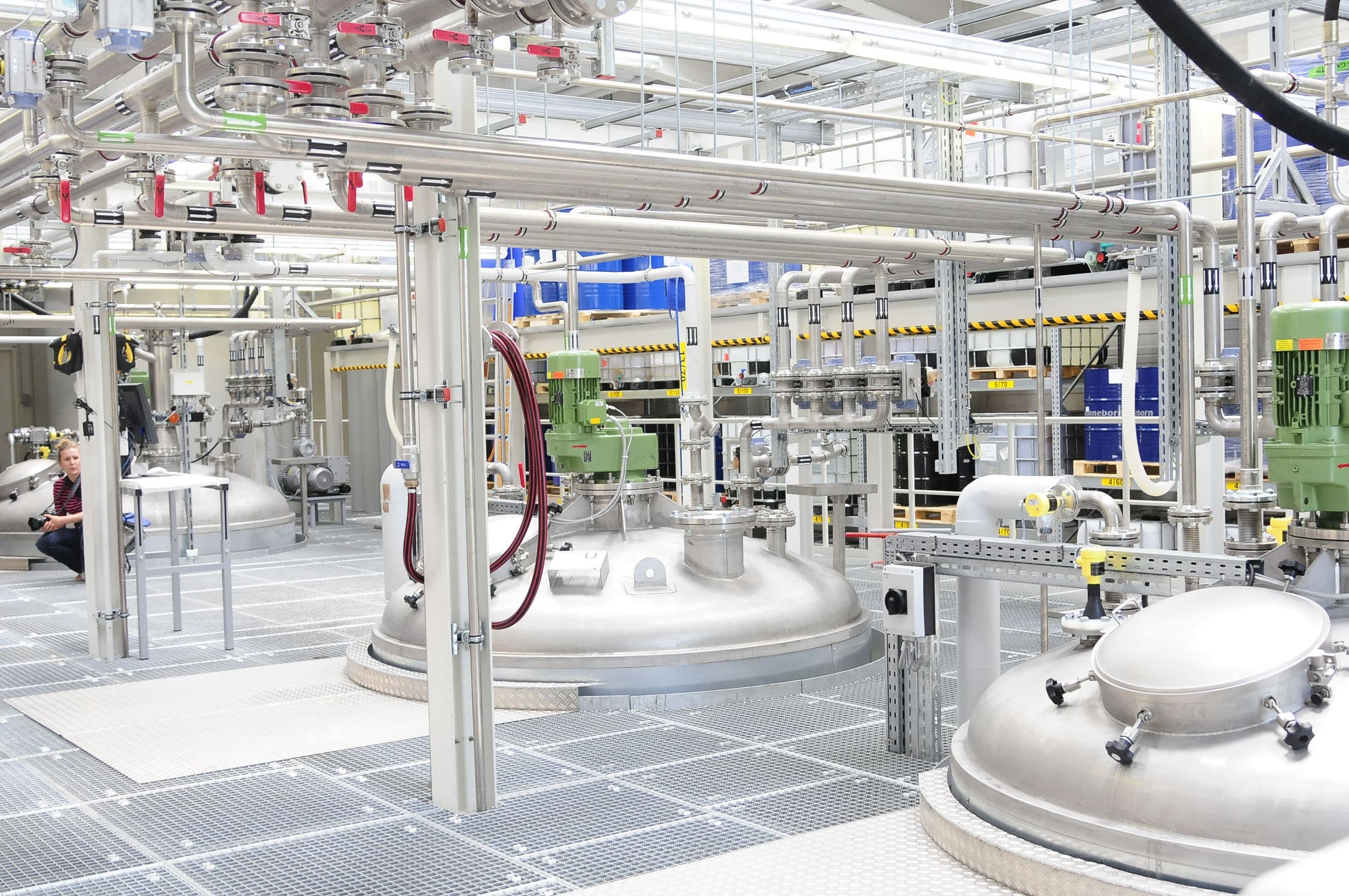
The weighing module of the weighing and dosing system application strictly manages the weighing process. The results after weighing raw and auxiliary materials are automatically read and printed with barcodes by the system from the weighing tool, and the entry information includes: raw and auxiliary material name, raw and auxiliary material batch number, raw and auxiliary material expiration date, raw and auxiliary material quantity (or weight), and finished product batch used for feeding or replenishment, etc.; the weighing process and the results are displayed in the interface of the weighing room and checked with the production tasks. The weighing process and results are displayed on the interface of the weighing room and checked with the production tasks, and an alarm message is generated when the checking does not match. At the same time, the system provides unified management and prompts for the calibration and alignment of weighing scales.
Electronic Batch Record: The EBR module automatically generates electronic batch records that meet the requirements of GMP and FDA, and the dosage system realizes real-time tracking and recording of process parameters, alarms, and over-limit handling of the product production process and operators, which is convenient for future historical tracking, and meets the requirements of GMP, which is: real-time factual recording of the whole process of production operations. Hurricane o蛖 complete production batch record contains real-time data collected from the production batching and control system (process parameters, batching status, operating parameters, environmental parameters), laboratory test results, weighing data, material inventory, material consumption in the production process, records of manual operation (clearing, checking), and automatically carry out the calculation of yield and material balance. At the end of the production batch, the dosage system provides on-line electronic batch record approval function, the system can automatically capture deviation events (while supporting manual entry of deviation events and commentary).
Production process tracking. Track various elements of the production process, including material tracking, personnel tracking and dosage tracking.
FDA-compliant electronic signatures. The dosing system meets the requirements for electronic records and electronic signatures to ensure the validity and reliability of electronic data.
Production Reports. Utilizing the real-time massive data generated in the business system, the actual completion of the production, dosage, quality and material of the operation process is statistically and analytically analyzed from a historical perspective.
The entire MES dosage plant architecture is compliant with industry standards such as S95/88 simplifying the integration between the business system (ERP), the manufacturing execution system (MES) and the automation layer. The J2EE-based platform provides support for third-party content, enabling dosage system integrators and IT departments to easily extend the functionality of PharmaSuite.
The dosage system enables the optimal allocation of resources, online monitoring and data collection of the manufacturing system, real-time monitoring and management of water and raw material ingredients, and the ability to automatically detect whether the product meets the requirements of the U.S. FDA standards. In addition, the realization of electronic records of the production process of each batch of products, in the domestic industry, solves the problem of the company's manufacturing process and the ERP system of the information silo, through the interface, realizes the production order instantly issued and the immediate feedback of the production of the work report, instead of manually issued work orders and manually enter the work report data, which greatly improves the efficiency of the work; through the immediate feedback of the work report data, it greatly facilitates the scheduling personnel and the commercial staff. Through the instant feedback of work report data, it greatly facilitates the scheduling personnel and business personnel to understand the processing degree of work orders, and improves the efficiency of information communication.
Through the MES mixer, the workshop production basically reaches the material management refinement, standardization of operating procedures, dosage operation visualization and personnel management information.
In terms of material management, all materials have their own sub-batch code, and need to be scanned and double-checked when they are used, which is bound to the production batch and eliminates the risk of mixing materials. In terms of protocol operation, all operations must complete the current operation before the next operation, which improves the staff's concentration on the current work. At the same time, the standards of all operations are indicated in the MES system, and operations that do not meet the standards can not be executed, so that employees are forced to comply. The operating parameters of the dosage system are all read by themselves and displayed in the MES system, which makes it easier for the operators to grasp the situation of the dosage operation, and abnormalities of the dosage can be detected at the first time. At the same time, all operators must go through MES training and apply for registration of rights before they can operate, with clear allocation of rights and detailed personnel information, which facilitates the scheduling of workshop production.
As a customized solution oriented to the customer's needs, the final operating effect of the MES dosage system is inseparable from the design and implementation of the system. In terms of GMP validation compliance, Batching provides a system acceptance program and a computerized system validation program compliant with GAMP5, which ensures GMP validation compliance of MES Batching and validation of the implementation of the system.
Through the introduction of advanced MES manufacturing information management system, the batching enhances the enterprise's informationization level and production efficiency, reduces product manufacturing costs, and achieves the goals of compliance with international standards, high efficiency and quality, and circular development. The demonstration role after the project implementation is obvious, MES batching is one of the automation towards the "Connected Enterprise", "Connected Enterprise" goal is through the integration of information technology (IT) and operational technology (OT) to obtain operational, business and transaction data, and make full use of these data to improve the batching and batching. The goal of the Connected Enterprise is to capture operational, business and transactional data through the integration of information technology (IT) and operational technology (OT), and to fully utilize this data to improve ingredient and supply chain performance. For ingredients, the "Connected Enterprise" will greatly promote the technological upgrading of the pharmaceutical industry, strengthen the pharmaceutical product manufacturing process control and quality online testing and monitoring, significantly improve production efficiency, improve the overall competitiveness of enterprises in the market, and promote the sustainable development of the enterprise's goals.